blog
Leveraging data to boost yields and optimise processes in pharmaceutical manufacturing
Jan 24, 2022

Many pharmaceutical manufacturers still rely on batch testing and processing. This is a reliable method of production, but it is often a slow and inefficient one.
Manufacturing pharmaceutical products often involves multiple stages, carried out using different equipment. It’s an effective process, but not necessarily the most efficient way to do things. In all, the manufacturing process for one batch of a pharmaceutical product may take as long as a month, and at every stage, all the equipment used for the other stages is standing dormant.
According to the FDA, the pharmaceutical industry may be losing as much as USD 45 billion every year because of the general inefficiencies involved in manufacturing using the standard batch processing – delays, contamination and recalls.
In the US, the Food and Drug Administration is trying to persuade pharma companies to adopt process analytical technology (PAT) to change this and has implemented a regulatory framework to this end. The goal of PAT is for manufacturers to identify which aspects of their manufacturing processes directly affect the critical quality attributes of their products. These critical process parameters, once identified, can be closely monitored – ideally online and in real time – to increase efficiency, enhance consistency and reduce wastage.
FDA encourages continuous manufacturing methods
The FDA is also encouraging pharma companies to switch over to continuous manufacturing (CM), where raw materials pass through a continuous, uninterrupted manufacturing chain right up to the finished product. The FDA claims that CM improves product quality and helps to reduce shortages in medicine. It also enhances efficiency in manufacturing, to the point that a drug that might take a month to produce in batch processing can take as little as one day using a CM setup.
However, without rigorous testing of individual batches, it is necessary to have continuous quality monitoring and control to ensure that the pharmaceutical products maintain their safety and efficacy. This requires sophisticated second-level control systems to monitor the system to ensure that quality and safety are maintained and to promptly issue alarms in the case of any deviations.
But creating a system like this that can identify and monitor the critical process parameters or recreate the rigour of batch processing with the efficiency of continuous manufacturing is far from a simple proposition. Not all manufacturers have the capabilities or expertise to achieve this. However, a solution is available.
Qualifying AI Algorithms in Pharmaceutical Manufacturing
Pharmaceutical manufacturers generate vast amounts of data about their processes and testing, especially for biopharmaceutical processes that can run uninterrupted for weeks. These datasets represent a treasure trove of crucial information that can be utilised by advanced machine learning and data analytics technologies, making it possible both to optimise production and to rapidly identify anomalies.
The experts at Elisa IndustrIQ are experienced at working with huge datasets like these, cleaning them and ensuring that their formatting is consistent, as well as identifying and extracting features. Once the datasets are ready, the modelling can begin.
Advanced machine learning algorithms analyse the data, learning what trends and features correspond to real-life outcomes: What features and parameters affect the efficiency and yield of each phase of the manufacturing? Which behaviours in the parameters are indicators of an anomaly or deviation in production?
The outcome of this process is a detailed description of those parameters and features that result in improved yields. This allows pharmaceutical companies to predict exactly what conditions will result in optimal efficiency in their manufacturing processes, and this increased efficiency means more effective use of resources as well as the maximum possible manufacturing yield. And of course, every improvement in efficiency comes with a corresponding boost to the sustainability of manufacturing.
Through an iterative development process, Elisa IndustrIQ can also create sophisticated models of the manufacturing processes. These models can be used to spot anomalies and deviations almost as soon as they start to appear – and sometimes even before they become a problem.
Empowering teams with collaboration and visual dashboards
Elisa works closely with each customer to create visual dashboards tailored for their specific needs and processes. This provides an easy-to-follow overview of the key parameters and features that need to be monitored. The dashboard also issues alerts if it spots a value or a trend that indicates a manufacturing problem. This allows manufacturers to eliminate problems and get back to uninterrupted production as quickly as possible, resulting in increased yields, as well as giving them the ability to control their processes more efficiently.
As Antti Liski, Lead Data Scientist at Elisa, explains, “Working in close collaboration with the customer, we can develop a system that leverages their existing data sources and databases to help them get the best out of their processes. It’s not unreasonable to expect boost in yield in the region of 6% to 7% a year. But the visual dashboard is equally important. It gives our customers an intuitive overview of the key parameters and features of their manufacturing processes, letting them see the current situation at a glance, and allowing them to easily monitor trends over time to spot any deviations as quickly as possible, as well as giving them more effective control over processes, leading to improvements in quality and yield. And it’s possible to achieve all of this in a remarkably short period of time.”
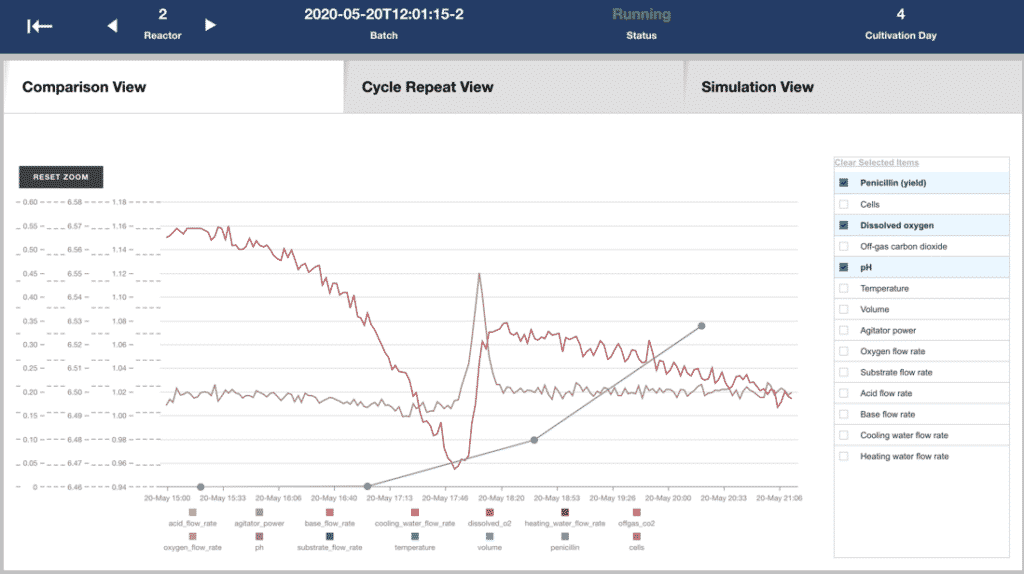
Picture: Real-time monitoring of process measurements
Customer case: A large European pharmaceutical manufacturer
This approach has already been proven to work, as the experts from Elisa have worked with a large European pharmaceutical manufacturer that wanted to develop a monitoring solution for their bioreactors. This customer already had over a decade’s worth of manufacturing data, and they had three goals for the project:
- Optimise the yield from their bioreactor process
- Learn to identify anomalies during their 50-day manufacturing process
- Develop the capacity to monitor a large number of process parameters in real time
The Elisa team got to work analysing, cleaning and formatting the dataset to identify the parameters and features that affected the yield of the bioreactor, as well as the values and trends that could indicate an anomaly or deviation in the manufacturing process.
First, they were able to produce a report detailing the factors the customer could pay attention to for increased yield from their bioreactor process. Next, taking a lean, iterative approach to development, the team were able to refine a model that monitors an array of parameters, watching for trends and values that indicate that an issue may be arising. All of this information was presented to the key stakeholders in the project using visualisations and layouts that made it easy for them to see how they could operationalise the results of the project. Finally, in order to help the customer going forward, they produced a tailored visual dashboard that presented the key details to the customer in a visual way that made it easy for the customer to turn the analytics findings into operational measures that improve their manufacturing process.
A final test proved the accuracy of the modelling and the abilities of the system – the customer checked the outcomes of the project against a validation dataset that the development team had not seen, and it passed the test with flying colours.
What’s more, thanks to sophisticated machine learning and the industry-leading expertise of the specialists from Elisa, the entire process took only three months. The development team worked closely with the customer right from the start to gain a deep understanding of the customer’s situation and needs. The development work was cloud-based to ensure scalability and a unified development environment.
Result: boosting yields and optimising processes
Within ninety days of the start of the project, Elisa was able to provide actionable insights to improve yield, a sophisticated model to monitor the manufacturing process, and a visual dashboard that makes it easy for them to oversee the process and issue alerts if a recognised problem arises – before it becomes a major issue.
“This customer’s situation presents an excellent showcase of the expertise that Elisa can bring to bear on projects like these. In only a short period of dedicated work, we were able to increase the yield from their bioreactor process and develop a monitoring system that also alerts the customer to potential problems in their processes. The customer already had all the data. We helped them to harness it for action”, says Jukka-Pekka Salmenkaita, Director of AI & Machine Learning at Elisa.
Conclusions
The combination of improved manufacturing efficiency and increased yield combined with the ability to spot and resolve problems extremely quickly not only helps in the production of existing pharma products – it may also even speed up the process of developing new treatments.
Immature pharmaceutical manufacturing processes tend to develop more anomalies and deviations. Spotting these as early as possible allows the problem to be fixed more quickly. But it is not only a question of putting out fires. If a specific anomaly occurs repeatedly, this is likely to indicate a problem in the process itself, rather than a quality deviation in the raw material. A system like the ones that Elisa IndustrIQ can create can monitor the anomalies that do occur, helping to identify and classify what kinds of problems these anomalies indicate. It can also help to determine which problems can be resolved by tweaking various production factors and parameters. All of this helps manufacturers get to a mature production process more quickly and efficiently, saving time and money.
If this kind of sophisticated, machine learning-powered monitoring systems were available during the development of the recent coronavirus vaccines, for example, the insights and increased efficiency that the system enables may have meant that these powerful and effective treatments could have been developed more quickly.
How To Improve Resilience And Grow Your Business Sustainably
Unexpected disruptive events in manufacturing always interrupt normal production conditions and cause production loss. How resilient are your planning and manufacturing processes? Are you prepared to deal with the unexpected?
Take the quiz below to find out how your organisation stacks up in the face of crisis and get expert insights on how to make your manufacturing more resilient.